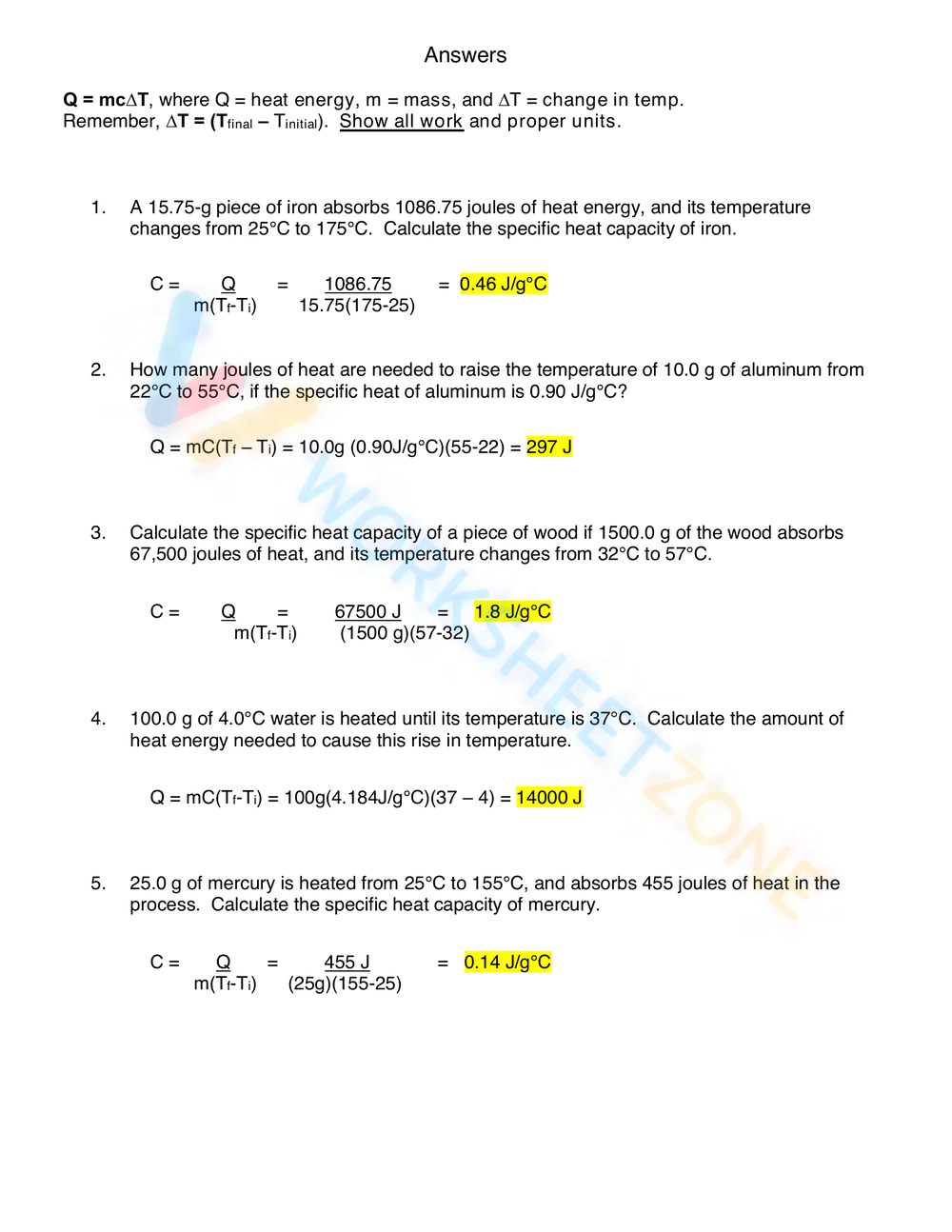
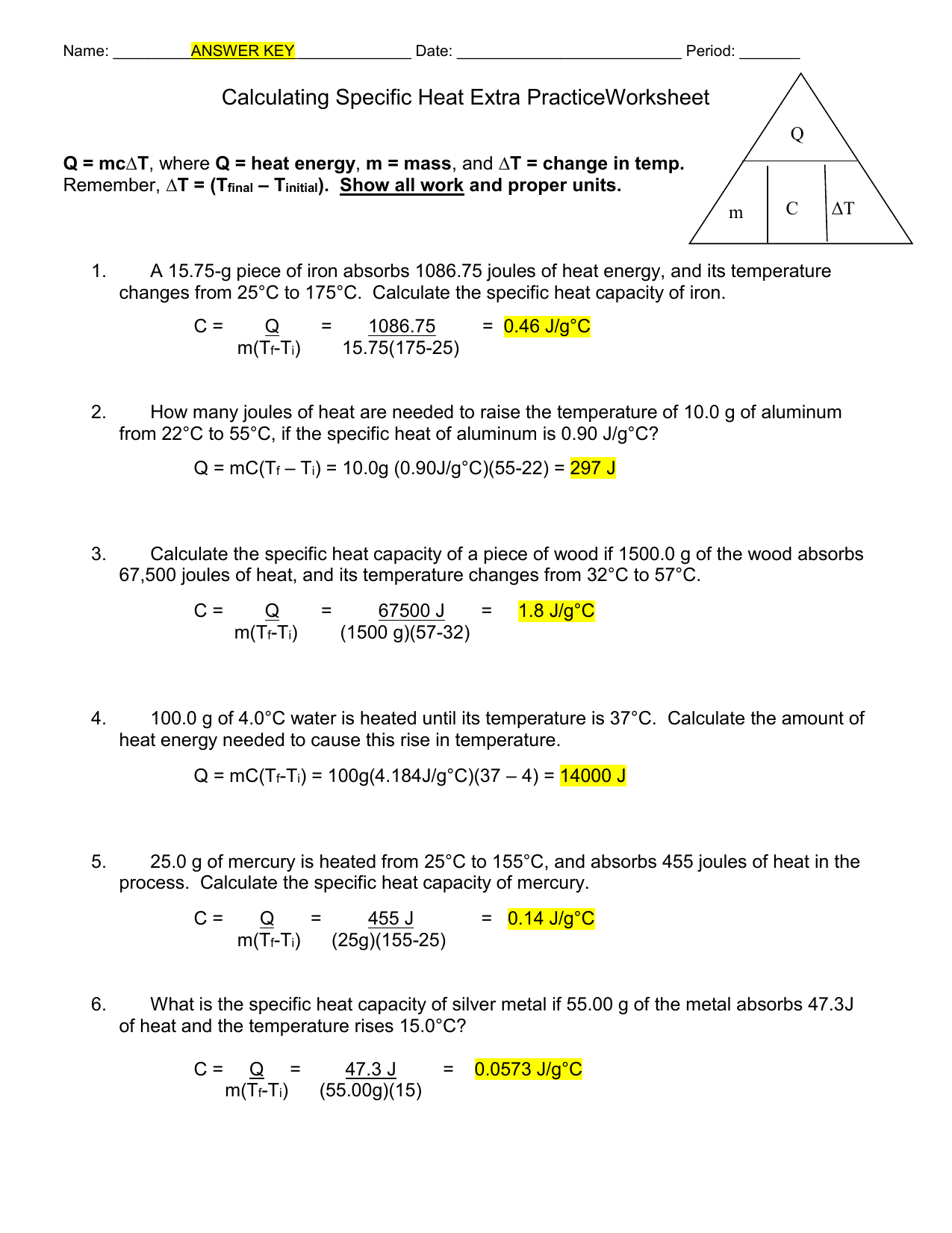
Calculating Specific Heat Extra Practice Worksheet - Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Show all work and units. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp.
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Use q = (m)(δt)(cp) to solve the following problems. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Show all work and units.
Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp.
Specific Heat Practice Worksheet at vangiavannablog Blog
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and units. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Use q = (m)(δt)(cp) to solve the following problems. Calculate.
Calculating Heat And Specific Heat Worksheets
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t.
Specific Heat Practice Worksheet Printable Word Searches
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Use q = (m)(δt)(cp) to solve the following problems. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculate the specific heat capacity of.
Calculating Specific Heat Extra Practice Worksheet
Use q = (m)(δt)(cp) to solve the following problems. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature.
Calculating Heat And Specific Heat Worksheet
Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of.
Calculating Heat And Specific Heat Worksheets
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Use q = (m)(δt)(cp) to solve the following problems. Calculate the specific heat capacity of.
Calculating Specific Heat Extra Practice Worksheet
Use q = (m)(δt)(cp) to solve the following problems. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculate the specific heat capacity of.
Extra practice calculating specific heat worksheet answers Studocu
Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculating heat specific heat is defined as the amount of.
Heat Capacity and Specific Heat Calculations Practice A 1 g
Show all work and units. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Use q = (m)(δt)(cp) to.
Calculating Specific Heat Extra Practice Worksheet Doc Template pdfFiller
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Show all work and units. Calculate the specific heat capacity of a piece of wood.
Show All Work And Units.
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculate the specific heat capacity of a piece of wood if 1500 g of the wood absorbs 67,500 joules of heat, and its temperature changes from to. Calculating heat specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Use q = (m)(δt)(cp) to solve the following problems.