Heisenberg Uncertainty Principle Worksheet - The heisenberg uncertainty principle part 1: Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. Heisenberg's uncertainty principle quiz for 11th grade students. Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Find other quizzes for chemistry and more on quizizz for free!
The heisenberg uncertainty principle part 1: Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. Find other quizzes for chemistry and more on quizizz for free! Heisenberg's uncertainty principle quiz for 11th grade students. Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of.
The heisenberg uncertainty principle part 1: Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. Find other quizzes for chemistry and more on quizizz for free! Heisenberg's uncertainty principle quiz for 11th grade students.
SOLVEDState the Heisenberg uncertainty principle and what it implies
Find other quizzes for chemistry and more on quizizz for free! Heisenberg's uncertainty principle quiz for 11th grade students. Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. The heisenberg uncertainty principle part 1: Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with.
Uncertainty Principle Heisenberg uncertainty principle
Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. The heisenberg uncertainty principle part 1: Heisenberg's uncertainty principle quiz for 11th grade students. Find other quizzes for chemistry.
1 Heisenberg Uncertainty Principle
The heisenberg uncertainty principle part 1: Heisenberg's uncertainty principle quiz for 11th grade students. Find other quizzes for chemistry and more on quizizz for free! Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with.
Heisenberg Uncertainty Principle Optics and Modern Physics Solved
The heisenberg uncertainty principle part 1: Find other quizzes for chemistry and more on quizizz for free! Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Heisenberg's uncertainty.
SOLVED Heisenberg’s uncertainty principle tells us that some
Heisenberg's uncertainty principle quiz for 11th grade students. Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Find other quizzes for chemistry and more on quizizz for free! The heisenberg uncertainty principle part 1: Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is.
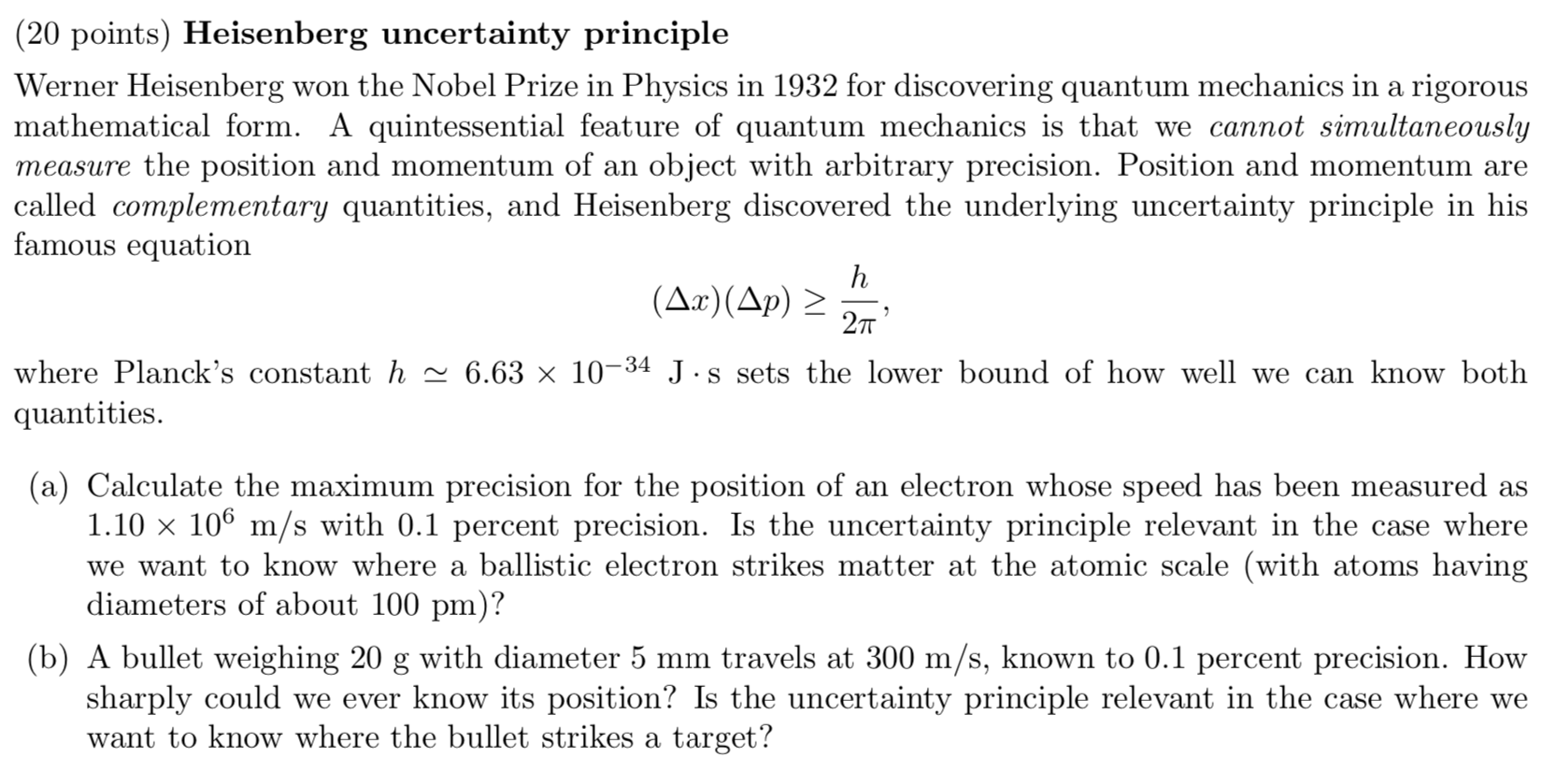
Solved (20 points) Heisenberg uncertainty principle Werner
Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. The heisenberg uncertainty principle part 1: Heisenberg's uncertainty principle quiz for 11th grade students. Find other quizzes for chemistry.
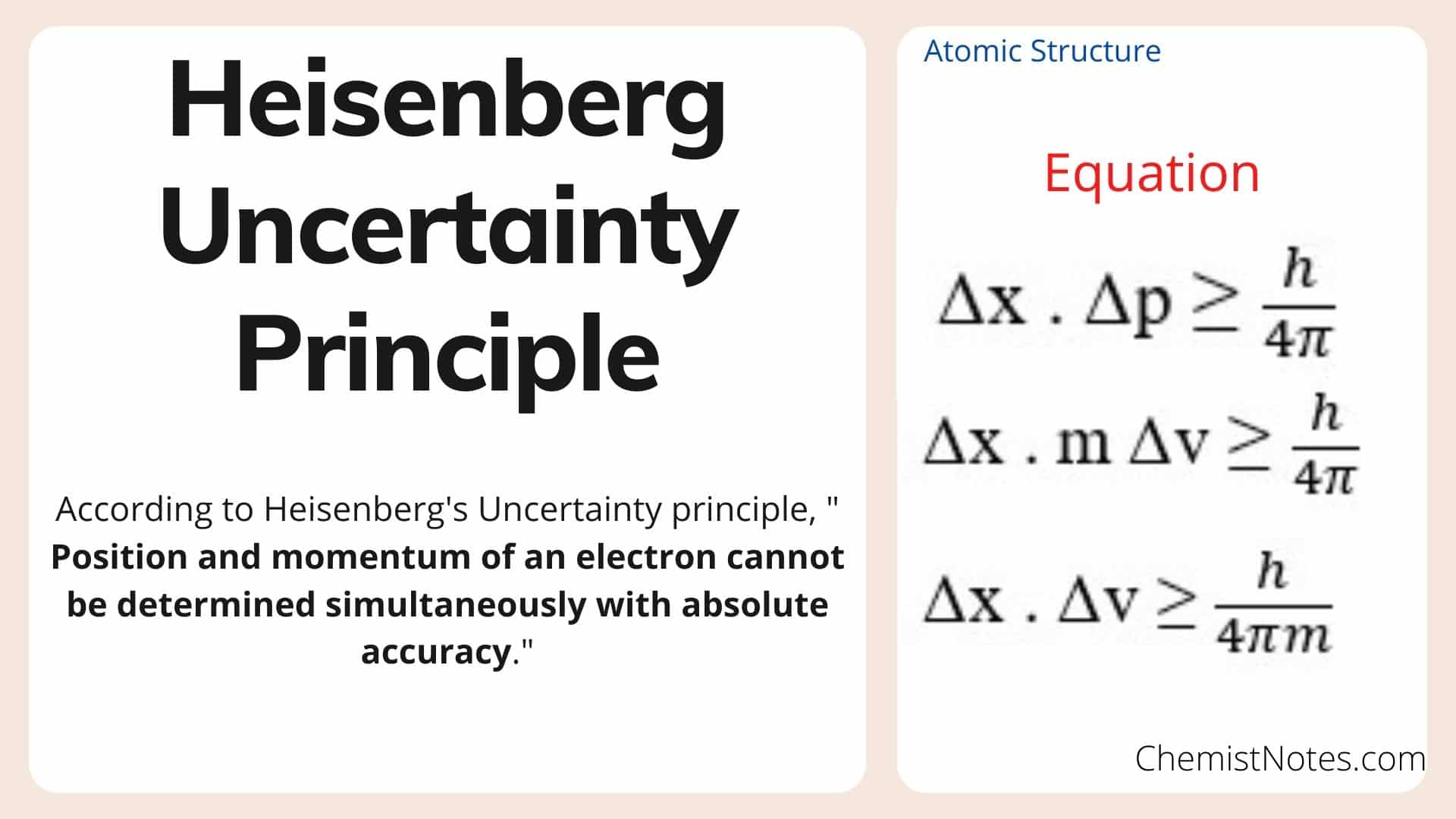
Heisenberg Uncertainty Principle Definition, Equation, and Application
Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Heisenberg's uncertainty principle quiz for 11th grade students. The heisenberg uncertainty principle part 1: Find other quizzes for chemistry.
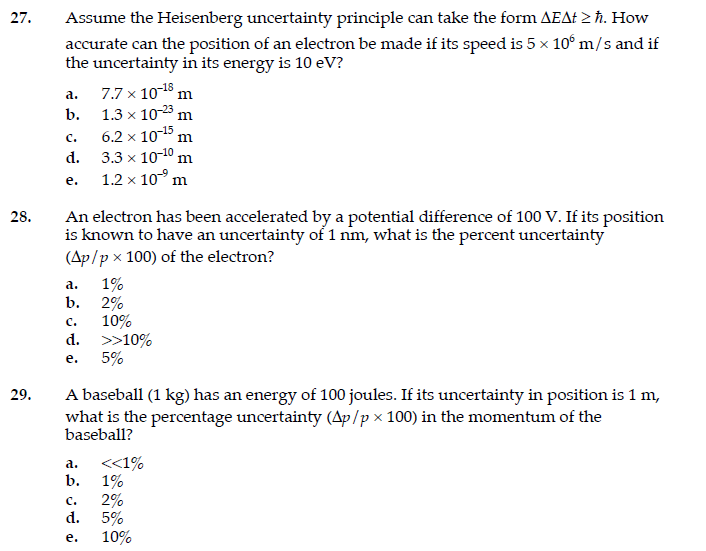
Solved Assume the Heisenberg uncertainty principle can take
Find other quizzes for chemistry and more on quizizz for free! Heisenberg's uncertainty principle quiz for 11th grade students. The heisenberg uncertainty principle part 1: Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is.
Heisenberg Uncertainty Principle Quiz & Worksheet for Kids
Heisenberg's uncertainty principle quiz for 11th grade students. Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. The heisenberg uncertainty principle part 1: Find other quizzes for chemistry and more on quizizz for free! Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is.
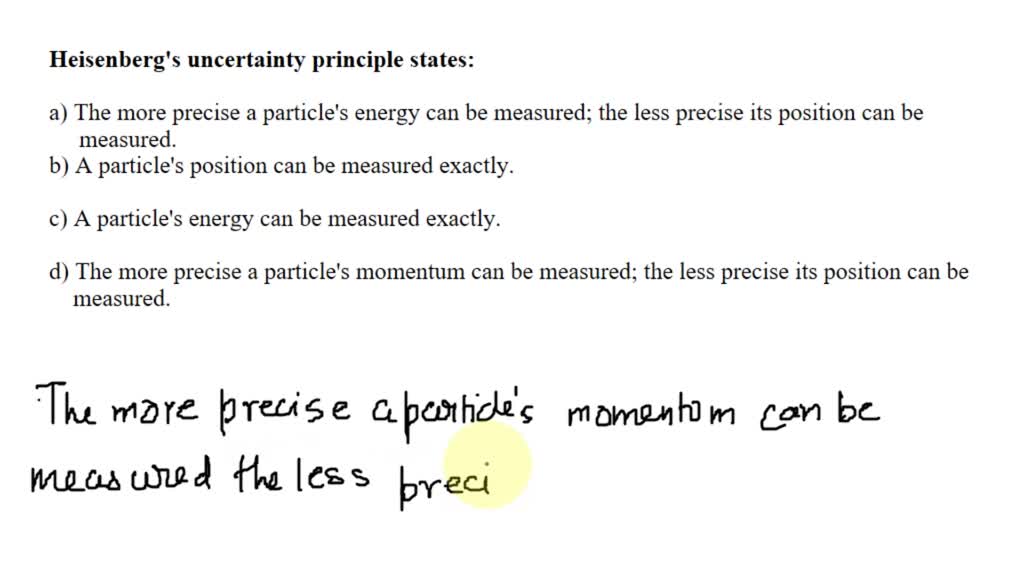
SOLUTION Heisenberg uncertainty principle handwritten notes Studypool
Find other quizzes for chemistry and more on quizizz for free! Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. The heisenberg uncertainty principle part 1: Using heisenberg's uncertainty principle, calculate the approximate uncertainty in the position of an electron with the uncertainty in the speed of. Heisenberg's uncertainty.
Using Heisenberg's Uncertainty Principle, Calculate The Approximate Uncertainty In The Position Of An Electron With The Uncertainty In The Speed Of.
Werner heisenberg proposed the heisenberg uncertainty principle in 1927, which states that it is impossible to measure simultaneously the exact. Find other quizzes for chemistry and more on quizizz for free! Heisenberg's uncertainty principle quiz for 11th grade students. The heisenberg uncertainty principle part 1: