Monitoring Plan Template For Clinical Trials - Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Throughout the template there are suggested. Clinical monitoring and data management plan (cmp/dmp) checklist. Welcome to global health trials' tools and templates library. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. This template is a suggested format for a monitoring plan developed by tb survey teams. Niams has guidelines and templates to help. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Please note that this page has been updated for 2015 following a quality check.
Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Welcome to global health trials' tools and templates library. Clinical monitoring and data management plan (cmp/dmp) checklist. Niams has guidelines and templates to help. Please note that this page has been updated for 2015 following a quality check. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. This template is a suggested format for a monitoring plan developed by tb survey teams. Throughout the template there are suggested. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format.
The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. Clinical monitoring and data management plan (cmp/dmp) checklist. This template is a suggested format for a monitoring plan developed by tb survey teams. Welcome to global health trials' tools and templates library. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Throughout the template there are suggested. Please note that this page has been updated for 2015 following a quality check. Niams has guidelines and templates to help.
Clinical Trial Project Management Plan Template
How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Please note that this page has been updated for 2015 following a quality check. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Clinical monitoring and data management plan (cmp/dmp) checklist. Throughout.
Monitoring Plan Template For Clinical Trials
How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Please note that this page has been updated for 2015 following a quality check. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. This template is a suggested format for a monitoring.
Monitoring Report Template Clinical Trials
The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. Welcome to global health trials' tools and templates library. This template is a suggested format for a monitoring plan developed by tb survey teams. Throughout the template there are suggested. Niams has guidelines and templates to.
Clinical Trial Monitoring Plan Template
Niams has guidelines and templates to help. Welcome to global health trials' tools and templates library. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Throughout the template there are suggested. Clinical monitoring and data management plan (cmp/dmp) checklist.
A Centralized Monitoring Approach Using Excel For The within Monitoring
Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Welcome to global health trials' tools and templates library. Niams has guidelines and templates to help. Throughout the template there are suggested. Clinical monitoring and data management plan (cmp/dmp) checklist.
Medical Monitoring Plan Template
The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality check. Investigators should consider using this template when developing the data and safety monitoring.
Monitoring Report Template Clinical Trials
Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. This template is a suggested format for a monitoring plan developed by tb survey teams. Clinical monitoring and data management plan (cmp/dmp) checklist. Welcome to global health trials' tools and templates library. Throughout the template there are suggested.
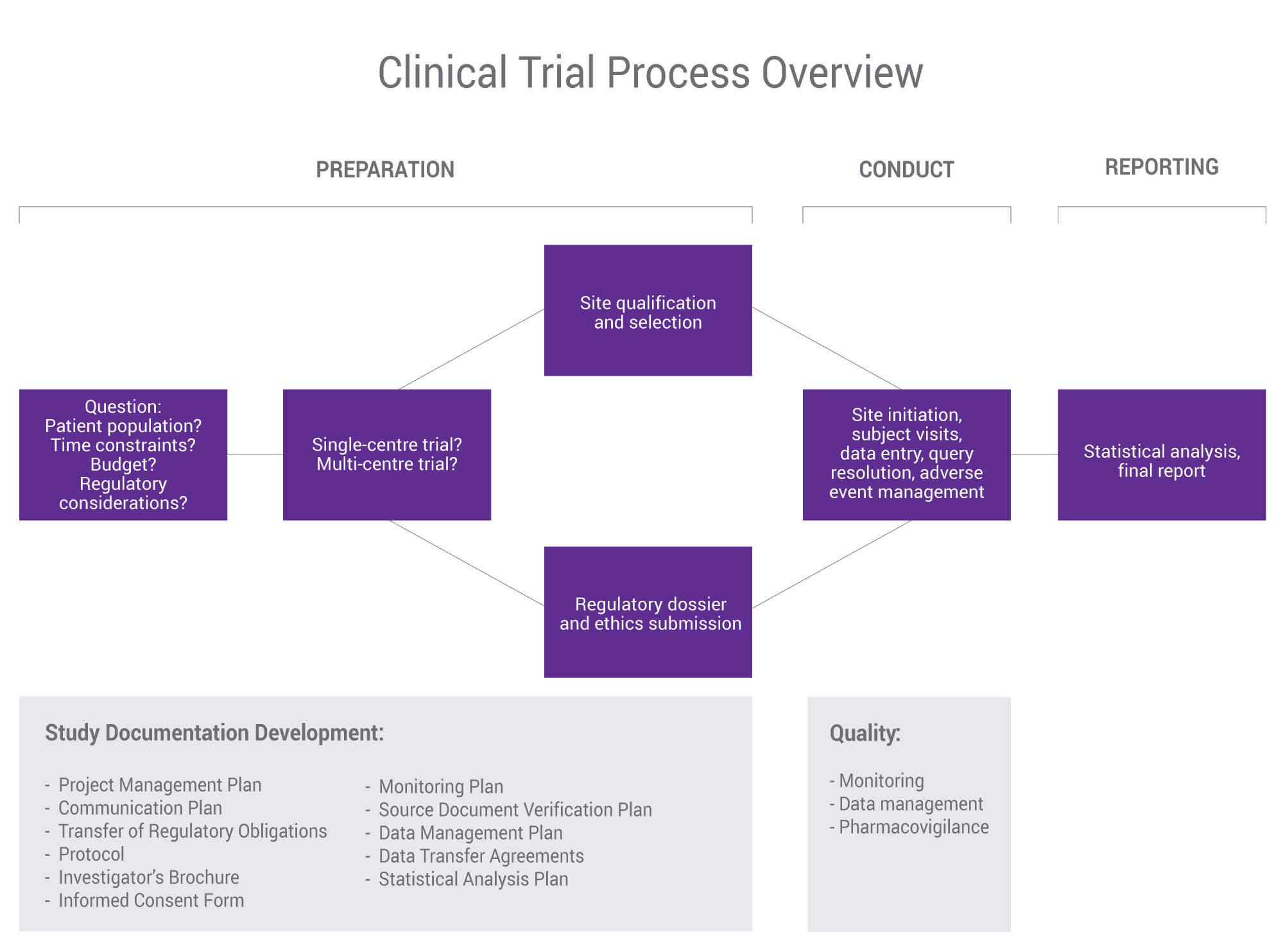
3.8. Template for monitoring plan
This template is a suggested format for a monitoring plan developed by tb survey teams. Clinical monitoring and data management plan (cmp/dmp) checklist. Niams has guidelines and templates to help. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Throughout the template there are suggested.
Management Plans Al Trial Project Plan Example Template with regard to
Clinical monitoring and data management plan (cmp/dmp) checklist. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Welcome to global health trials' tools and templates library. Niams has guidelines and templates to help. Throughout the template there are suggested.
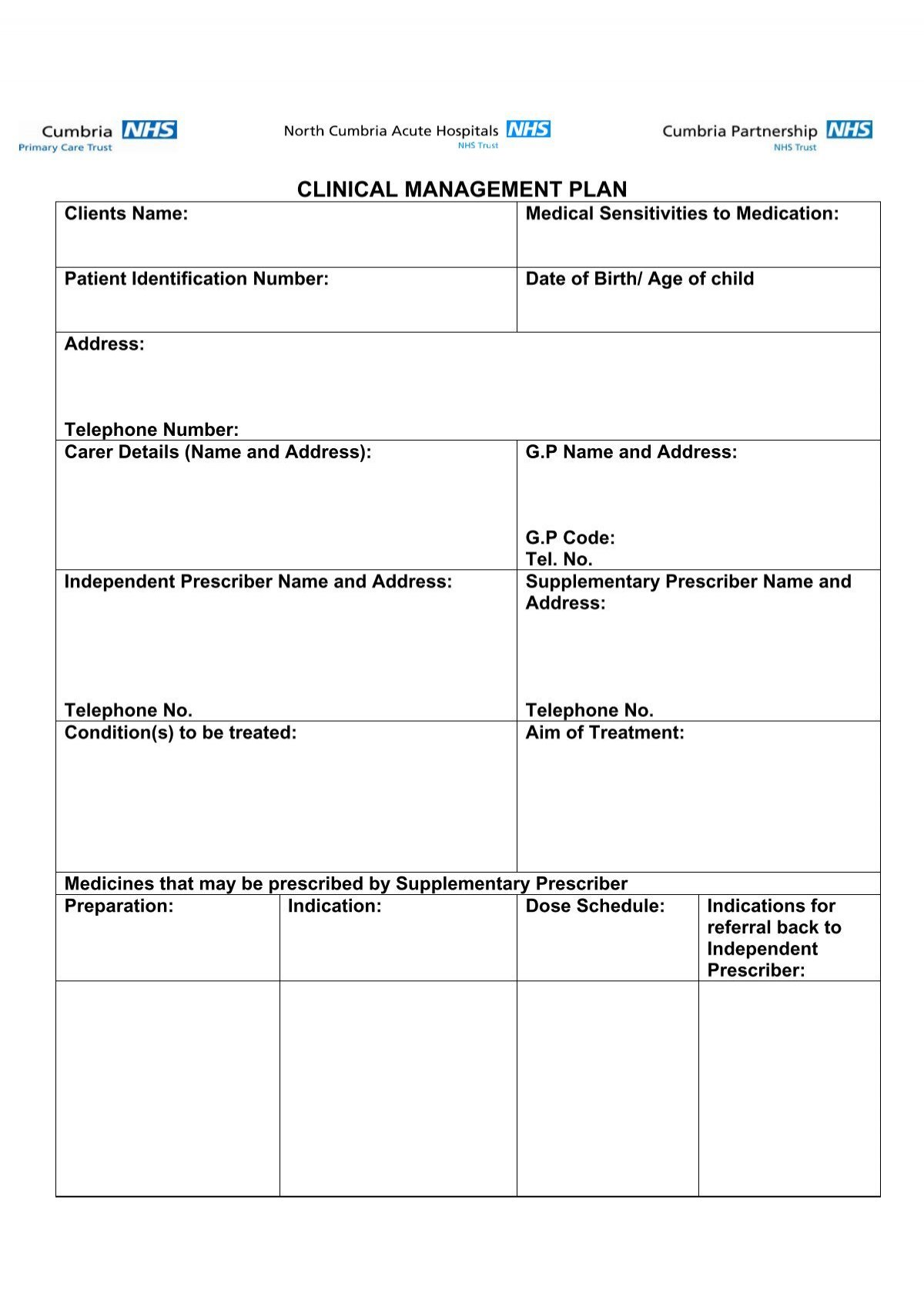
Clinical management plan template NHS Cumbria
The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format. Niams has guidelines and templates to help. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study. Throughout the template there are suggested. Clinical monitoring and.
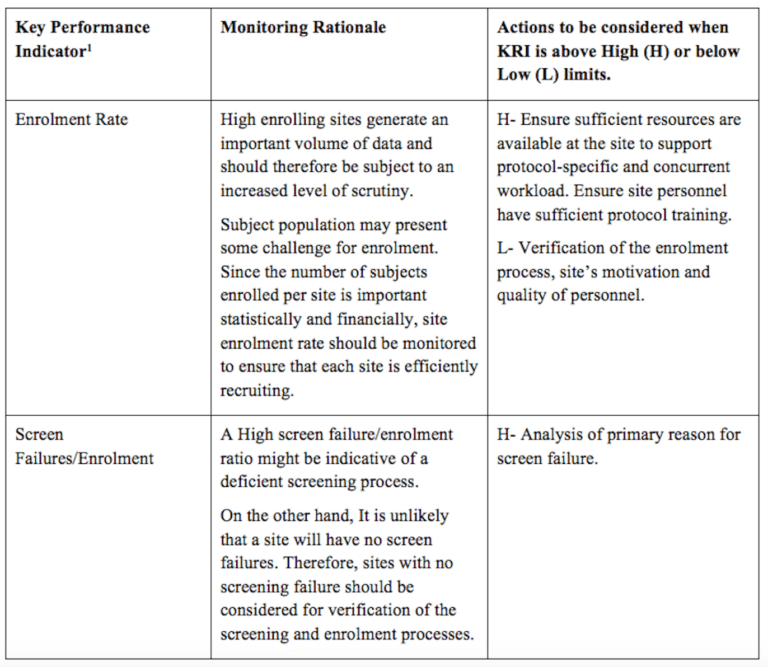
Clinical Monitoring And Data Management Plan (Cmp/Dmp) Checklist.
Welcome to global health trials' tools and templates library. This template is a suggested format for a monitoring plan developed by tb survey teams. Investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. The dsmp may be developed using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format.
Please Note That This Page Has Been Updated For 2015 Following A Quality Check.
Niams has guidelines and templates to help. Throughout the template there are suggested. How and when monitoring will be done should be documented in a monitoring plan prior to activation of the study.