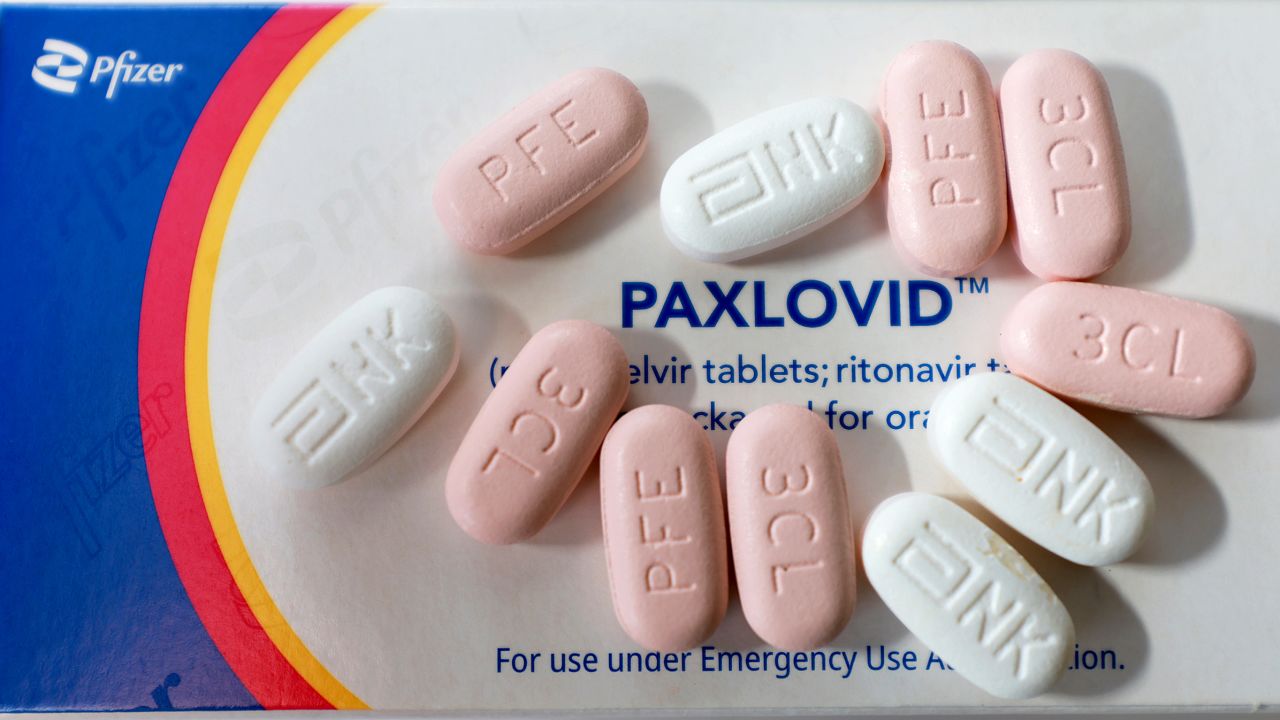
Paxlovid Emergency Use Authorization Fact Sheet - Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
Paxlovid Skippack Pharmacy
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
Frequently Asked Questions on the Emergency Use Authorization for
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
Nirmatrelvirritonavir (Paxlovid™) Drug interactions with psychiatric
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
Paxlovid prescription available via Ontario pharmacists Dec. 12
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
Dr. Fauci talks COVID after Pfizer's Paxlovid treatment
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
A Comparison Of Paxlovid Versus Molnupiravir The First Oral COVID
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
Paxlovid Doesn’t Work For Healthier Patients, Pfizer Says Time
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
COVID pill Paxlovid gets full FDA approval after more than a year of
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.
FDA approves Paxlovid to treat Covid19 CNN
Food and drug administration (fda) has issued an emergency use authorization (eua) to make paxlovid available for the.





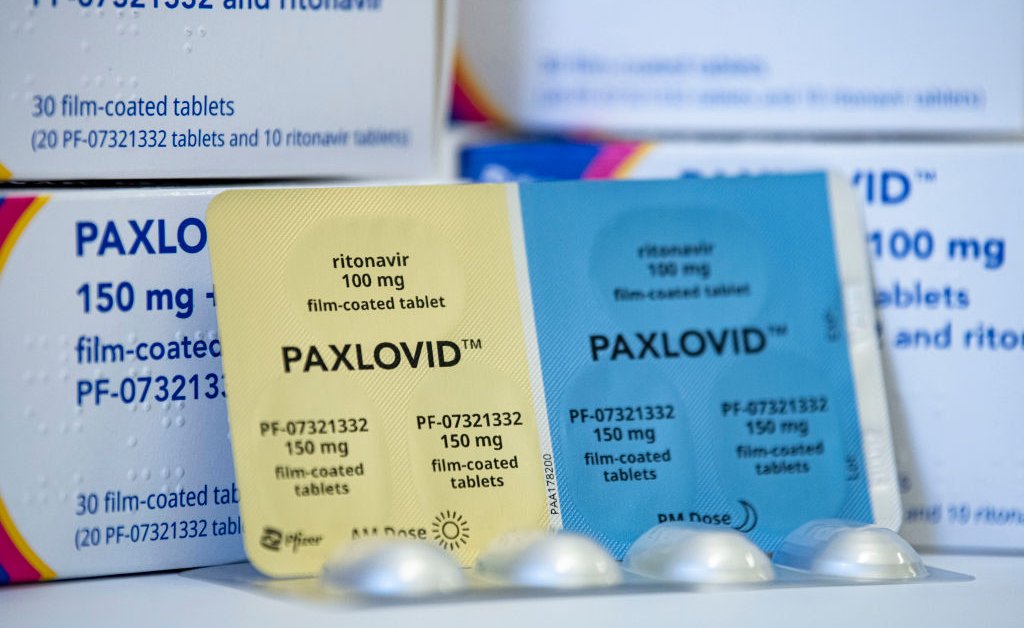
/cloudfront-us-east-1.images.arcpublishing.com/gray/IBGPW3A56ZEYBJKNIPN73MPSRY.jpg)