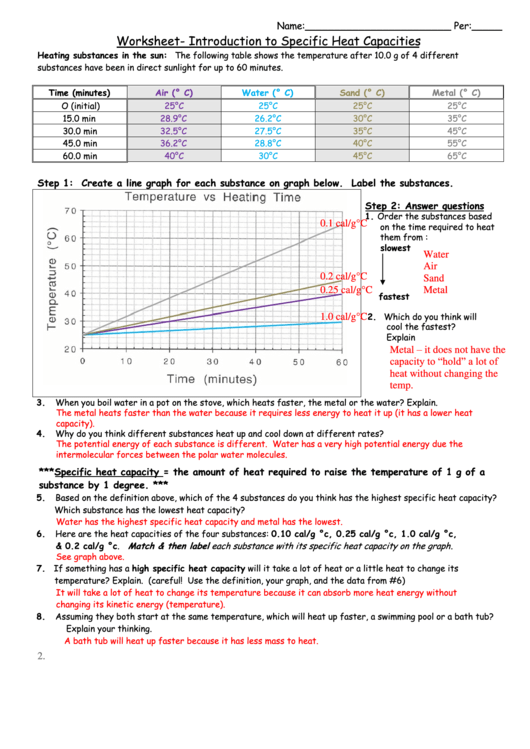
Specific Heat Chemistry Worksheet - For q= m c δ t : Identify each variables by name & the units associated with it. C = specific heat capacity (the amount of energy required to raise 1 gram of a substance 1°c; Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Every substance has a unique specific heat capacity. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Calculate the heat capacity of. Every substance has its own specific.
C = specific heat capacity (the amount of energy required to raise 1 gram of a substance 1°c; Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. For q= m c δ t : Identify each variables by name & the units associated with it. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Use q = (m)(δt)(cp) to solve the following problems. Every substance has its own specific. Every substance has a unique specific heat capacity. Calculate the heat capacity of. Show all work and units.
Every substance has a unique specific heat capacity. Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Use q = (m)(δt)(cp) to solve the following problems. Identify each variables by name & the units associated with it. Calculate the heat capacity of. Every substance has its own specific. For q= m c δ t : C = specific heat capacity (the amount of energy required to raise 1 gram of a substance 1°c; Show all work and units.
Specific Heat Worksheet
Every substance has a unique specific heat capacity. For q= m c δ t : Every substance has its own specific. Use q = (m)(δt)(cp) to solve the following problems. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp.
Worksheet Introduction To Specific Heat Capacities Winters Chemistry
C = specific heat capacity (the amount of energy required to raise 1 gram of a substance 1°c; Show all work and units. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Use q = (m)(δt)(cp) to solve the following problems. Specific heat is defined as.
Specific Heat Practice Worksheet Printable Word Searches
Calculate the heat capacity of. For q= m c δ t : Every substance has a unique specific heat capacity. Use q = (m)(δt)(cp) to solve the following problems. Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c.
Specific Heat Capacities Worksheet PDF Heat Chemistry
Calculate the heat capacity of. For q= m c δ t : Identify each variables by name & the units associated with it. Every substance has its own specific. Use q = (m)(δt)(cp) to solve the following problems.
Specific Heat Worksheet Answers 1
For q= m c δ t : Use q = (m)(δt)(cp) to solve the following problems. Every substance has a unique specific heat capacity. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and units.
Specific Heat Worksheet Chemistry
Every substance has a unique specific heat capacity. Calculate the heat capacity of. Identify each variables by name & the units associated with it. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Specific heat is defined as the amount of heat (in _____) needed to.
Chemistry Specific Heat Worksheet
Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Use q = (m)(δt)(cp) to solve the following problems. C = specific heat capacity (the amount of.
Solved Specific Heat and Heat Capacity Worksheet DIRECTIONS
For q= m c δ t : Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Every substance has its own specific.
Specific Heat Problems Worksheet Answers —
Identify each variables by name & the units associated with it. C = specific heat capacity (the amount of energy required to raise 1 gram of a substance 1°c; Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c. Every substance has its own specific. Use q = (m)(δt)(cp) to.
For Q= M C Δ T :
Calculate the heat capacity of. Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Specific heat is defined as the amount of heat (in _____) needed to raise ____ gram of a substance ____˚c.
Every Substance Has A Unique Specific Heat Capacity.
Identify each variables by name & the units associated with it. Every substance has its own specific. Calculating specific heat extra practice worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. C = specific heat capacity (the amount of energy required to raise 1 gram of a substance 1°c;