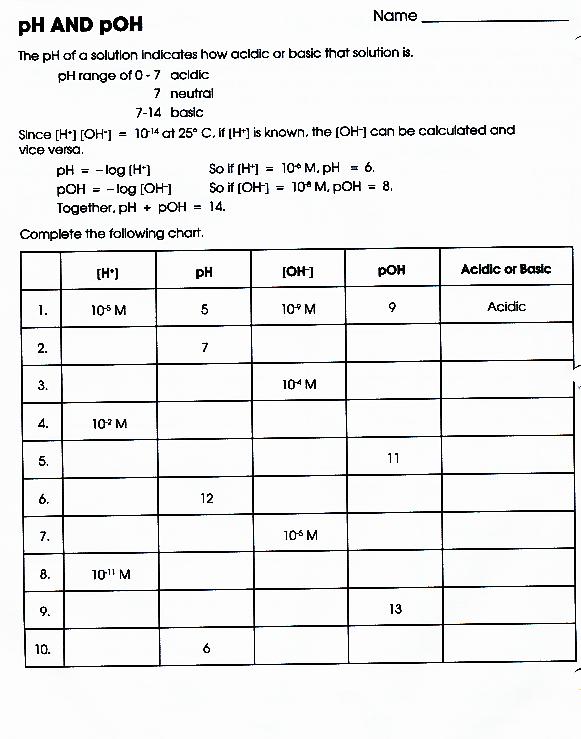
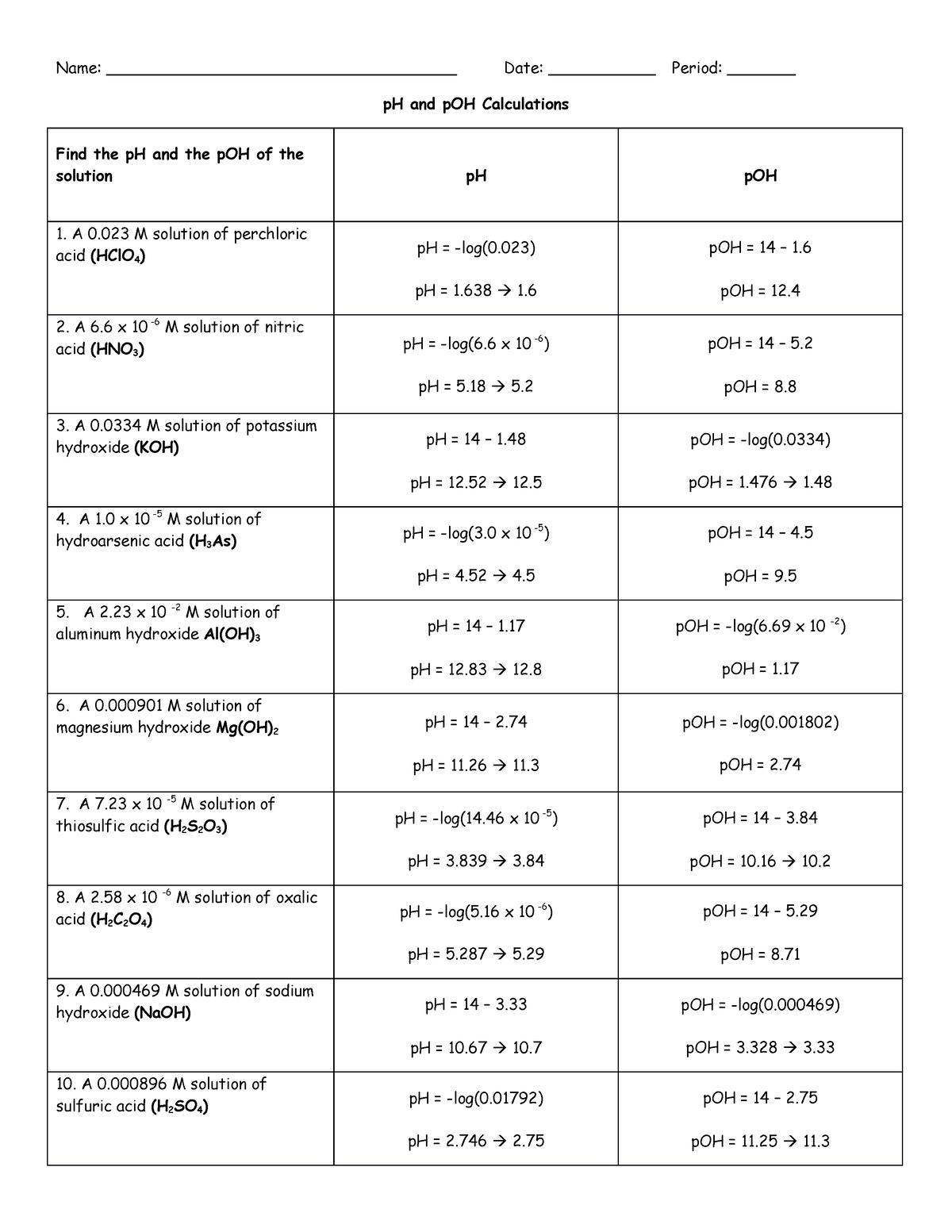
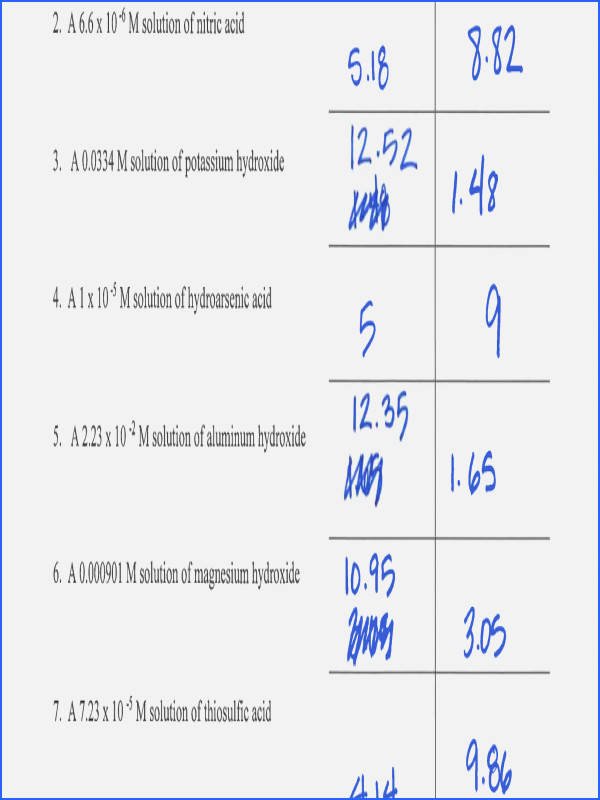
Worksheet Ph And Poh Calculations - What are the ph and poh of the resulting solution? Use mental math shortcuts for calculating ph and poh to solve the problems below. What is the concentration of [h+] in a solution whose ph = 4.3? Solve the following ph calculations. Write the formula, plug numbers into formula, & give answer with correct units and significant. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. If you get stuck, try the logs and antilogs practice 1 worksheet. What is the ph of a solution that has a hydronium concentration of 3.4 x 10.
If you get stuck, try the logs and antilogs practice 1 worksheet. What are the ph and poh of the resulting solution? Solve the following ph calculations. Use mental math shortcuts for calculating ph and poh to solve the problems below. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Write the formula, plug numbers into formula, & give answer with correct units and significant. What is the concentration of [h+] in a solution whose ph = 4.3?
What is the ph of a solution that has a hydronium concentration of 3.4 x 10. Use mental math shortcuts for calculating ph and poh to solve the problems below. What is the concentration of [h+] in a solution whose ph = 4.3? Solve the following ph calculations. What are the ph and poh of the resulting solution? Write the formula, plug numbers into formula, & give answer with correct units and significant. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. If you get stuck, try the logs and antilogs practice 1 worksheet.
pH and pOH Calculations Worksheet Worksheets Library
Use mental math shortcuts for calculating ph and poh to solve the problems below. If you get stuck, try the logs and antilogs practice 1 worksheet. What are the ph and poh of the resulting solution? What is the concentration of [h+] in a solution whose ph = 4.3? What is the ph of a solution that has a hydronium.
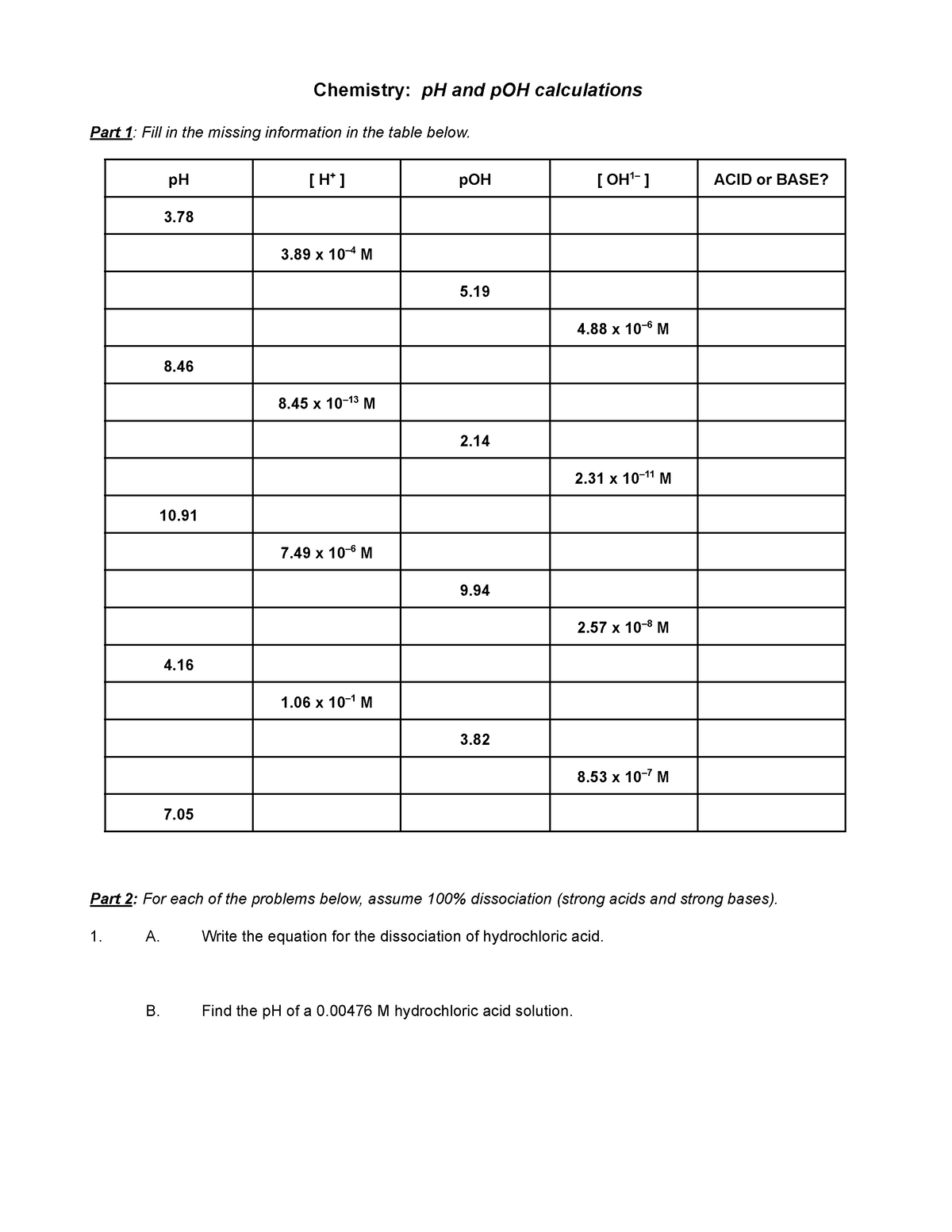
07c p H problems worksheet Chemistry pH and pOH calculations Part 1
What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. What are the ph and poh of the resulting solution? Use mental math shortcuts for calculating ph and poh to solve.
pH and pOH Practice Worksheet
Solve the following ph calculations. What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What are the ph and poh of the resulting solution? What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Use mental math shortcuts for calculating.
Ph And Poh Worksheet
If you get stuck, try the logs and antilogs practice 1 worksheet. What are the ph and poh of the resulting solution? Solve the following ph calculations. Use mental math shortcuts for calculating ph and poh to solve the problems below. What is the concentration of [h+] in a solution whose ph = 4.3?
Calculating Ph And Poh Worksheet
What is the ph of a solution that has a hydronium concentration of 3.4 x 10. What is the concentration of [h+] in a solution whose ph = 4.3? Write the formula, plug numbers into formula, & give answer with correct units and significant. If you get stuck, try the logs and antilogs practice 1 worksheet. Solve the following ph.
Solved pH and pOH Calculations Worksheet 1 15 Determine
What are the ph and poh of the resulting solution? Solve the following ph calculations. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph = 11.50. Write the formula, plug numbers into formula, & give answer with correct units and significant. If you get stuck, try the logs.
Worksheet Ph Calculations Worksheet Ph Calculations Answers
Write the formula, plug numbers into formula, & give answer with correct units and significant. Use mental math shortcuts for calculating ph and poh to solve the problems below. Solve the following ph calculations. What are the ph and poh of the resulting solution? What mass of naoh should be added to 300.0 ml of water in order to prepare.
SOLUTION Chemistry Ph and Poh Calculations Study Guide Studypool
What is the concentration of [h+] in a solution whose ph = 4.3? What are the ph and poh of the resulting solution? Write the formula, plug numbers into formula, & give answer with correct units and significant. Solve the following ph calculations. Use mental math shortcuts for calculating ph and poh to solve the problems below.
Ph Calculations Worksheet With Work
If you get stuck, try the logs and antilogs practice 1 worksheet. Use mental math shortcuts for calculating ph and poh to solve the problems below. What are the ph and poh of the resulting solution? Write the formula, plug numbers into formula, & give answer with correct units and significant. Solve the following ph calculations.
Ph And Poh Calculations Worksheet 2
What are the ph and poh of the resulting solution? What is the ph of a solution that has a hydronium concentration of 3.4 x 10. If you get stuck, try the logs and antilogs practice 1 worksheet. What mass of naoh should be added to 300.0 ml of water in order to prepare a solution with a ph =.
What Mass Of Naoh Should Be Added To 300.0 Ml Of Water In Order To Prepare A Solution With A Ph = 11.50.
If you get stuck, try the logs and antilogs practice 1 worksheet. Use mental math shortcuts for calculating ph and poh to solve the problems below. What are the ph and poh of the resulting solution? What is the concentration of [h+] in a solution whose ph = 4.3?
Write The Formula, Plug Numbers Into Formula, & Give Answer With Correct Units And Significant.
Solve the following ph calculations. What is the ph of a solution that has a hydronium concentration of 3.4 x 10.
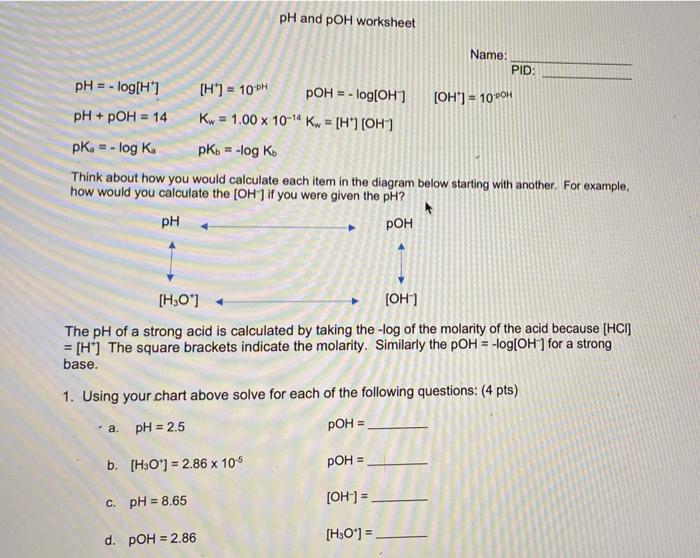
/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)